MAY 2024 - IP UPDATE
- May 21, 2024
- Newsletter
- MAY 2024 NEWSLETTER
.png)
USPTO UPDATES
USPTO Proposes New Fees and Increases that Encourage Compact Prosecution
BY ANDREW M. OLLIS
The USPTO recently proposed significant new fees and fee increases for fiscal year 2025. See 89 Fed. Reg. 23226 (April 3, 2024), available here. The proposed rulemaking spans more than 100 pages, potentially affects 455 existing fees, and proposes 73 new fees.
If the proposals are adopted, many applicants will be incentivized to rethink multiple aspects of their prosecution strategy. Notably affected areas include filing Requests for Continued Examination (RCEs), continuation applications, including more than 20 claims in an application, and filing large Information Disclosure Statements (IDSs). According to the USPTO, the proposed fee changes promote compact prosecution and taking actions earlier in prosecution rather than later.
The proposed fee changes are grouped into three categories: (A) an across-the-board fee increase, (B) front end fee changes, and (C) targeted fee changes. See 89 Fed. Reg. at 23235. For category (A), all fees not otherwise raised are proposed to be increased by approximately 5%. For category (B), so-called “front end” fees (e.g., filing, search, and examination fees) are targeted to increase by 10%. For category (C), the proposed fee changes appear aimed to change applicant behavior and/or address perceived imbalances between current fees and costs to the USPTO for carrying out the corresponding actions.
Some of the more notable proposed fee increases and new fees (for undiscounted entities) are summarized below:
Continuing Applications. New fees are proposed to be added for filing a continuing application in which a benefit claim is presented more than 5 years ($2,200 fee) or more than 8 years ($3,500 fee) after the earliest benefit date. See 89 Fed. Reg. at 23237, Table 5. This would be in addition to the fees for filing the continuing application itself. The USPTO indicated that it seeks to encourage more efficient prosecution and recapture lost maintenance fee revenue. See 89 Fed. Reg. at 23238.
Excess Claim Fees. The fees for each claim in excess of 20 are proposed to double from $100 to $200. The cost for each independent claim in excess of 3 is proposed to increase from $480 to $600. See 89 Fed. Reg. at 23241, Table 7. The USPTO is clearly attempting to discourage the filing of extra claims.
Information Disclosure Statements (IDSs). The proposal creates a new tiered fee structure for IDSs, with a new fee being proposed to start after a total of 50 references are submitted during prosecution ($200) and increasing fees after a total of 100 references ($500) and 200 references ($800) are submitted. See 89 Fed. Reg. at 23243, Table 9. The proposed fee structure appears designed to discourage submissions of large IDSs.
Requests for Continued Examination (RCEs). Another significant proposed set of fee increases is directed to RCEs. See 89 Fed. Reg. at 23247, Table 12. The fee for filing a first RCE is proposed to increase from $1,360 to $1,500. The fee for filing a second RCE is proposed to jump 25%, from $2,000 to $2,500. Finally, the fee for a third RCE and beyond is proposed to increase dramatically, from $2,000 to $3,600. These fees discourage the filing of multiple RCEs and could encourage applicants to file an appeal earlier in prosecution. Appeal fees are proposed to increase by approximately 5%. See 89 Fed. Reg. at 23265.
Terminal Disclaimers. The proposal also introduces a new tiered set of fees designed to encourage the filing of Terminal Disclaimers early in prosecution. See 89 Fed. Reg. at 23248, Table 14. The fee for filing a terminal disclaimer before the first office action is proposed to increase from $170 to $200. After the first action and before a final action or allowance, the fee is proposed to increase to $500. After final action or allowance, the fee is proposed to increase to $800 and then to $1,100 if a Terminal Disclaimer is filed after a Notice of Appeal. After issuance or in reissue, the fee is proposed to increase from $170 to $1,400.
Other notable proposed fee increases and new fees include the following:
After Final Consideration Pilot Program 2.0 (AFCP 2.0). A new fee of $500 is proposed for a filing under the AFCP 2.0. See 89 Fed. Reg. at 23236. Currently there is no fee for filing an AFCP 2.0 Request.
Patent Term Extension (PTE). The PTE fee is proposed to increase substantially, from $1,180 to $6,700. See 89 Fed. Reg. at 23245.
Design Applications. Total fees for filing a design application are proposed to increase about 20%, from $1,080 to $1,300. See 89 Fed. Reg. at 23239.
Inter Partes Review (IPR) and Post Grant Review (PGR). Fees for IPRs and PGRs are proposed to increase a further 25% from current levels. See 89 Fed. Reg. at 23251-2, Table 16. If the proposed fees take effect, the fees for requesting and instituting an IPR would increase to $51,875, and fees for requesting and instituting a PGR would increase to $59,375.
Comments on the proposed rules must be received by June 3, 2024.
JPO UPDATES
JPO Held the Five Trademark Offices (TM5) Mid-Term Meeting
BY KASUMI KANETAKA
On April 23, 2024, the Japan Patent Office (JPO) held Five Trademark Offices (TM5) Mid-Term Meeting as the host office for this year’s meeting. The TM5 includes the JPO, the USPTO, the EUIPO, the CHIPA, and the KIPO.
TM5 is promoting to harmonize trademark practices and procedures, as well as to improve trademark services, and the participants discussed future goals and a new project proposal. Among the 15 cooperative projects, the JPO leads the following three projects: the “Bad Faith Trademark Project,” the “IT Support for Trademark Examination Project,” and the “User Involvement Project (with the EUIPO)”. The JPO reported a workshop result for the Bad Faith Trademark Project, an upcoming expert meeting for the IT Support for Trademark Examination Project, and a preparation for the TM5/INTA Joint Workshop for the User Involvement Project.
The 13th TM5 Annual Meeting, where TM5 will review this year's activities, is scheduled for December 9-11, 2024, in Hakone, Japan.
Please see the full report here.
JPO Releases Revision of the Examination Handbook on System for Non-Disclosure of Patent Applications
BY KASUMI KANETAKA
The JPO, on May 1, 2024, released information regarding the revision of examination guidelines for patent and utility models in Japan in accordance with “commencement of operation of the System for Non-disclosure of Patent Applications.” This is due to the Economic Security Promotion Act which came into effect on the same day. It is noted that the revisions do not establish any new policies or procedures beyond what is stipulated in the Act.
Please see our March 2024 Newsletter discussing the System for Non-Disclosure of Patent Applications, and please see here for the full report.
Invention Day in Japan
BY KASUMI KANETAKA
Besides celebrating World Intellectual Property Day on April 26 every year, the JPO celebrates the 18th of April as Invention Day in Japan. The Invention Day was set as the Patent Monopoly Act was announced on that day in 1885. Please see here for a brief history of the patent system in Japan and a poster for Invention Day in 2024.
A Good Chance to Establish and Expand IP Portfolio in Japan for Foreign Entities
BY KASUMI KANETAKA
The yen has been weak against the US dollar for the past few years. Recently, it experienced a historical drop, recording 160 yen per US dollar. The record was after about three decades since the Lost Decades started in early 1990. Considering the patent prosecution fees in Japanese yen do not alter much, it is a good chance for foreign entities, especially U.S. entities, to establish and expand IP portfolios in Japan. Please see our March 2024 Newsletter showing the accelerated examination provided by the JPO and consider the business opportunities in Japan.
AI UPDATES
USPTO Publishes Request for Comments Regarding the Impact of the Proliferation of Artificial Intelligence on Prior Art
BY NICHOLAS ROSA, PHD
On April 30, 2024, the USPTO published a Request for Comments on the future impact of publications by generative AI on the issue of prior art. Previously, the USPTO has consistently come down against generative AI as an inventor, saying that “under current law, only natural persons may be named as an inventor in a patent application.” See Inventorship Guidance for AI-Assisted Inventions, 89 FR 10043, Docket No. PTO-P-2023-0043. Based on this stance, however, it is unclear if a publication by a generative AI could be prior art to a patent application. Toward providing an answer to that question, the USPTO is seeking public comments on “what qualifies as prior art, the assessment of the level of skill of a [person having ordinary skill in the art (PHOSITA)], and determinations of patentability made in view of these evaluations.”
The USPTO recognizes that generative AI has the potential to generate vast amounts of prior art. Many practitioners find such AI-generated publications to be of low quality, frequently being ambiguous, technically deficient, or nonsensical to the point of not actually advancing a useful art. However, both the USPTO and courts operate with a presumption that a public disclosure provides a description that enables the public to make and use the disclosure. The presumption does not currently take into account who or what made the disclosure. A flood of AI-generated publications can create a mountain of prior art that is time-consuming and costly for the USPTO to analyze, for a PHOSITA to reasonably locate, and for applicants to overcome (if it can be overcome). The USPTO is seeking input on how to address AI-generated content with various amounts of human input.
In addition, AI as a tool for an inventor has the ability to greatly expand the relative “skill” of a PHOSITA. As such, AI has the potential to affect multiple aspects of patent law including enablement and various facets of obviousness such as the reasonable expectation of success, obvious to try, the “analogous art” standard, and lack of predictability in the art. The USPTO is also seeking input on how to address the availability and use of AI as a tool for inventors. The published request, entitled “Request for Comments Regarding the Impact of the Proliferation of Artificial Intelligence on Prior Art, the Knowledge of a Person Having Ordinary Skill in the Art, and Determinations of Patentability Made in View of the Foregoing” (89 FR 34217, Docket No. PTO-P-2023-0044), is available here. Written comments must be submitted through the Federal eRulemaking Portal at www.regulations.gov using docket number PTO-P-2023-0044 and must be received on or before July 29, 2024
USPTO Publishes Comments Regarding Inventorship Guidance for AI-Assisted Inventions
BY SAMEER GOKHALE
On May 13, 2024, the USPTO completed receiving comments from industry regarding the guidance for AI-assisted inventions originally published on February 13, 2024. The guidance provides clarity for USPTO stakeholders and personnel on how the USPTO will analyze inventorship issues for AI systems. The guidance explains that the inventorship analysis should focus on human contributions and that AI-assisted inventions are not categorically unpatentable. The guidance provides procedures for determining whether a natural person provided a significant contribution to the invention. The guidance discusses the impact these procedures have on other aspects of patent practice.
Industry comments on the guidance was received from large industry players, such as Google, IBM, and Qualcomm. While many commenters praised the guidance as both recognizing the use of AI in innovation while properly focusing on the roles of humans in the innovation process, some commenters were critical that guidance treats AI differently than the use of any other tool. The comments can be found here.
LIFE SCIENCES NEWS
FTC Attacks More Orange Book Patents
BY RICHARD D. KELLY
In its April 30, 2024, press release, here, the FDA issued its second list of patents, 300, its challenging as improperly listed in the Orange Book. The letters were sent to:
- AstraZeneca and Novo Nordisk for obesity and type-2 diabetes injectable drugs;
- Boehringer Ingelheim, Covis Pharma, Glaxo-Smith Kline, Novartis Pharmaceuticals Corp., Teva Pharmaceutical Industries Ltd. and some of their subsidiaries for asthma and COPD inhalers;
- Amphastar Pharmaceuticals Inc. for a glucagon nasal spray to treat severe hypoglycemia in type-1 diabetics.
A review of a small sample of patents identified in Novo Nordisk warning letter revealed that the patents did not claim the device in combination with any specific drug nor was one described in the specification like the first letter in 2023.
The statute on listing patents in the Orange Book requires:
(viii) the patent number and expiration date of each patent for which a claim of patent infringement could reasonably be asserted if a person not licensed by the owner of the patent engaged in the manufacture, use, or sale of the drug, and that—
(I)
claims the drug[1] for which the applicant submitted the application and is a drug substance (active ingredient) patent or a drug product (formulation or composition) patent; or
(II)
claims a method of using such drug for which approval is sought or has been granted in the application.
Industry has requested unsuccessfully for the FDA to advise as to the scope of this requirement. The language in section D of the definition of a drug can easily be read as including the applicator of a drug. The FDA has provisions for combinations of an active drug with an applicator, combination product. The FDA has published a guide of what constitutes a combination product, here, which describes categories of combination products:
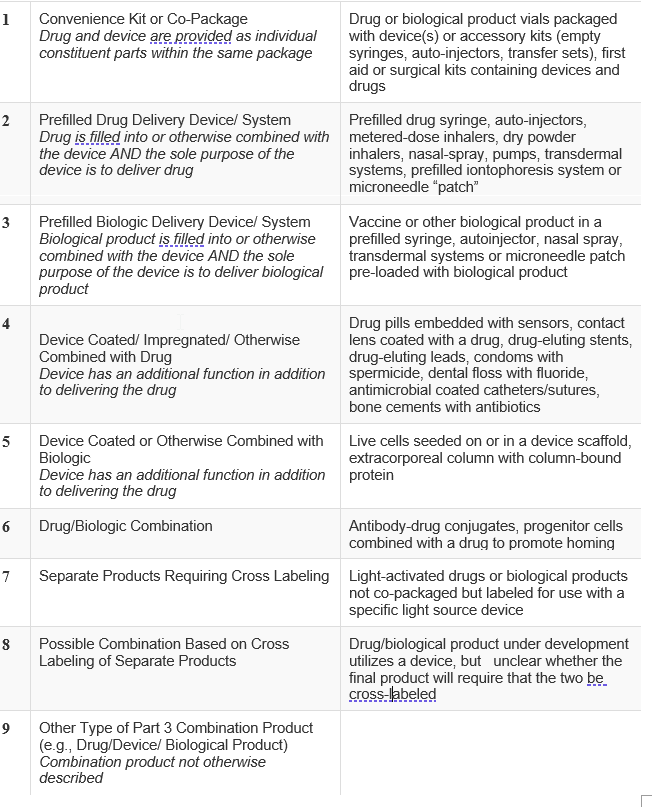
Given the FDA’s guidance, it appears that when the device is packaged with the drug or the device is sold prefilled with the drug, it should be ok. However, none of the patents attacked by the FTC appear to be drawn to or describe either of those concepts. To be safe, claims should be presented to one or both of these concepts which would fall squarely within the guidance. Claims to the device should also be presented in device patents going forward. Patentees should review their Orange Book listings for device patents to determine if they have such claims or if the claims a can be interpreted to include such limitations.
________________________
[1] (1) The term “drug” means (A) articles recognized in the official United States Pharmacopoeia, official Homoeopathic Pharmacopoeia of the United States, or official National Formulary, or any supplement to any of them; and (B) articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in man or other animals; and (C) articles (other than food) intended to affect the structure or any function of the body of man or other animals; and (D) articles intended for use as a component of any article specified in clause (A), (B), or (C). See 21 U.S. C. § 321(g)(1)









 Counseling & Strategic Advice
Counseling & Strategic Advice IP Transactions
IP Transactions Litigation
Litigation PTAB Proceedings
PTAB Proceedings Start-Up
Start-Up Technology Transfer
Technology Transfer Trademark & Designs
Trademark & Designs U.S. Patent Procurement (Application Drafting & Prosecution)
U.S. Patent Procurement (Application Drafting & Prosecution)







